Introduction: Recent studies have shown that clonal B cells derived from patients with chronic lymphocytic leukemia (CLL) have altered histone modification landscapes compared to B cells from healthy controls. We have also shown that the BCR signaling pathway impacts the chromatin landscapes of CLL B cells, implicating the interaction between oncogenic signaling and epigenetic machinery in CLL (Wang et al., Blood Cancer J. 2022; Wang et al., Cancer Res (2023) 83 (7_Supplement): 4725). However, the majority of the chromatin studies in CLL concentrate on the active enhancers associated with oncogene expression; there is a very limited understanding of lost enhancers in CLL: 1) What is the function of lost enhancers; 2) How are these lost enhancers maintained in CLL; 3) Can these lost enhancers be detected at the precursor state of CLL, monoclonal B-cell lymphocytosis (MBL); 4) if this aberrant chromatin signature affected by CLL treatment.
Methods: We systematically mapped the genome-wide histone modification profile (histone marks: H3K4me1, H3K4me3, H3K9me3, H3K27ac, H3K27me3, and H3K36me3) in 22 previously untreated CLL samples, 6 high count MBL samples, and peripheral blood B cells from 6 healthy donors by Cleavage Under Targets and Tagmentation (CUT&Tag). We also analyzed B cells from 8 patients before and after one year of continuous Bruton tyrosine kinase inhibitor (BTKi) ibrutinib treatment. The genomic regions with a decrease in both H3K4me1 and H3K27ac enrichment (analyzed by Deseq2, fold change > 1, p < 0.01) were named lost enhancers. We used CRISPR-mediated epigenetic editing to study the role of gained H3K9me3 on EBF1 gene expression.
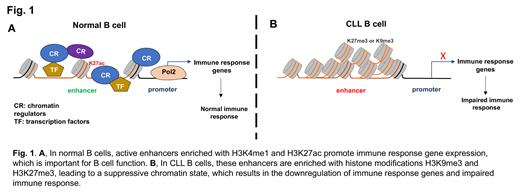
Results: Compared to normal B cells, the lost enhancers (genomic regions defined by decreased enrichment of H3K4me1 and H3K27ac) in CLL B cells showed increased heterochromatin marks (H3K9me3 and H3K27me3) enrichment. Importantly, these enhancers are associated with genes downregulated in CLL and involved in the immune response, indicating the source of epigenetic impact on impaired immunity in CLL. We further found that this aberrant chromatin structure also exits in clonal B cells from the precursor stage of MBL, implicating the importance of epigenetic silencing in CLL evolution. In particular, we found the lost enhancer signature across the gene locus EBF1, an important transcription factor that defines B cell lineage and regulates normal B cell activation. Using CRISPR-mediated epigenetic editing, we demonstrated that the gain of H3K9me3 is sufficient to silence the EBF1 enhancer and its gene expression. Finally, we found that while BTKi ibrutinib treatment partially inhibits the CLL-gained enhancers in some patients, it failed to restore the lost enhancers in CLL B cells.
Conclusion: In summary, we have elucidated an epigenetic silencing mechanism that may contribute to the loss of physiological function of normal B cells, which places the clonal B cells at a selection advantage during CLL evolution and increases the risk of serious infections in CLL and MBL ( Fig 1).
Disclosures
Parikh:Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Accerta Pharmaceuticals: Research Funding; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb-Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim Pharmaceuticals Incc: Membership on an entity's Board of Directors or advisory committees; Vincerx: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Research Funding; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees. Kay:Beigene: Membership on an entity's Board of Directors or advisory committees; Behring: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Vincerx: Research Funding; Sunesis: Research Funding; Pharmcyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Bristol Meyer Squib / Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; boehringer ingelheim: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal